Description
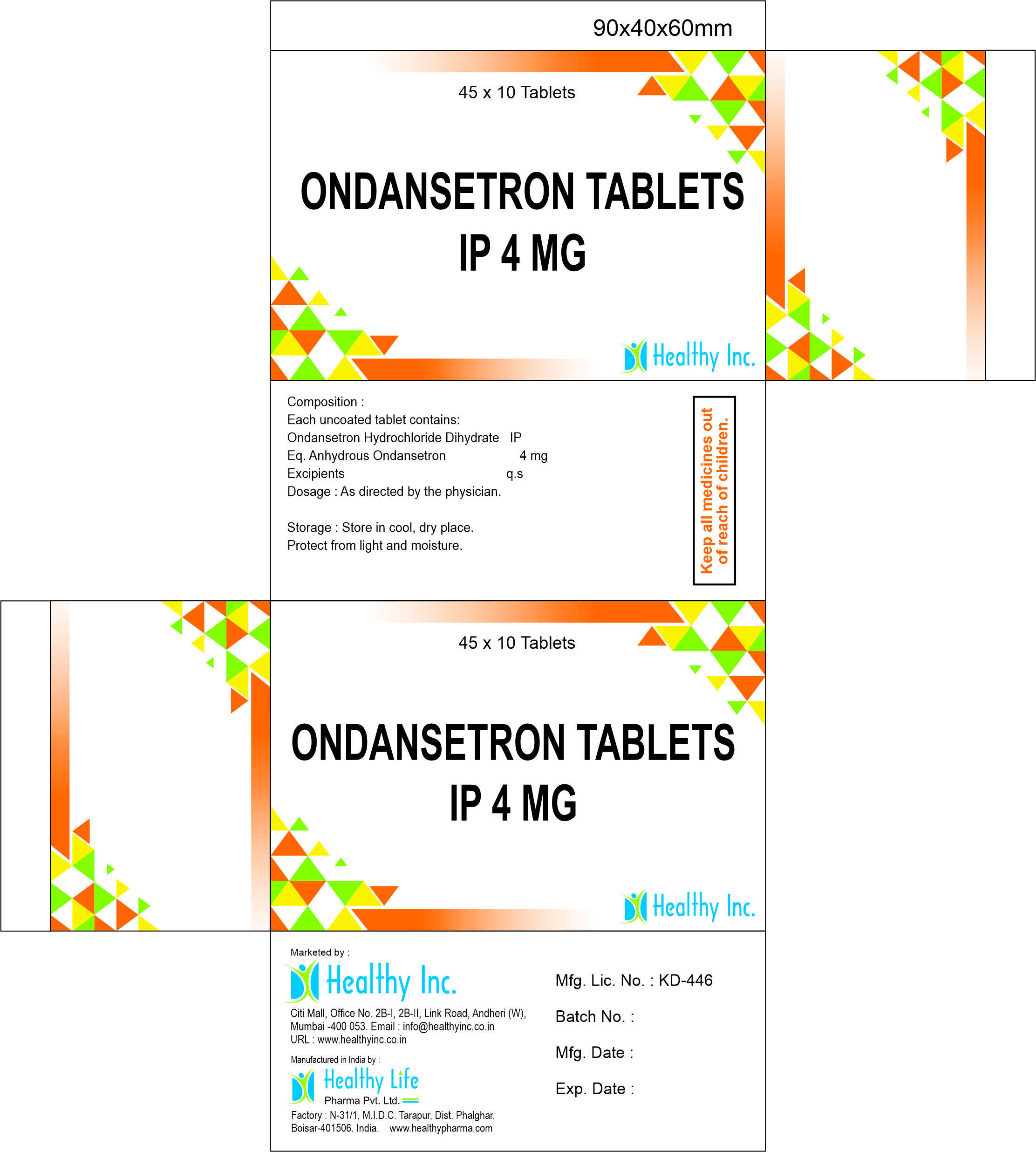
Ondansetron Tablets (4mg / 8mg)
Manufactured by: Healthy Life Pharma Pvt. Ltd. (WHO-GMP Certified)
Exported by: Healthy Inc (Star Export House)
1. Product Introduction
Healthy Life Pharma Pvt. Ltd. is a specialized Manufacturer of Ondansetron Tablets in India. Ondansetron is the “Gold Standard” 5-HT3 Receptor Antagonist used globally for the prevention and treatment of severe nausea and vomiting caused by Chemotherapy (CINV), Radiotherapy, and Surgery (PONV).
We offer Contract Manufacturing (Third Party) services for this critical antiemetic. Recognizing that nauseous patients often struggle to swallow water/pills, we specialize in manufacturing Mouth Dissolving (MD) / Orally Disintegrating Tablets (ODT). Our WHO-GMP certified facility in Mumbai utilizes advanced taste-masking and super-disintegrant technologies to ensure the tablet dissolves on the tongue in seconds without water. Healthy Inc manages the export logistics, supplying oncology centers and hospitals across the USA, Europe, and emerging markets.
2. Product Specifications
| Parameter | Specification |
| Product Name | Ondansetron Tablets / Mouth Dissolving Tablets |
| Generic Name | Ondansetron Tablets USP / BP / IP |
| CAS Number | 99614-01-4 (Ondansetron HCl Dihydrate) |
| Strength | 4mg / 8mg |
| Dosage Form | Film Coated Tablet (Yellow/White) / Mouth Dissolving (MD) |
| Standard | USP / BP / IP Compliant |
| Therapeutic Class | Antiemetic / 5-HT3 Antagonist |
| Shelf Life | 36 Months |
| Packaging | 10×10 Alu-Alu Blister (for MD) / 10×10 Blister |
3. Manufacturing Technology
Patient compliance during nausea is the formulation goal.
The Manufacturer: Healthy Life Pharma Pvt. Ltd.
Orally Disintegrating Technology (ODT): We formulate MD tablets using a matrix of super-disintegrants (like Crospovidone) and highly soluble excipients (Mannitol). This ensures the tablet disintegrates in < 30 seconds upon contact with saliva, requiring no water to swallow.
Taste Masking: Ondansetron is intensely bitter. We use Ion-Exchange Resins or specialized polymer coatings on the drug particles before compression. This masks the bitterness without slowing down the release of the drug in the stomach.
Friability Control: ODTs are naturally soft. We balance hardness and friability to ensure the tablets don’t break in the blister pack while being pushed out.
The Exporter: Healthy Inc
Alu-Alu Packaging: For MD tablets, moisture protection is critical (moisture makes them soft and sticky). We strictly use Alu-Alu Cold Form Blisters to ensure they remain crisp and effective.
4. Quality Assurance
We adhere to strict Pharmacopoeial standards:
Disintegration Time:
Standard Tabs: < 15 minutes.
MD Tabs: < 30 seconds (Benchmark for rapid relief).
Assay: We confirm the potency is strictly within 90-110% of the label claim.
Related Substances: We strictly monitor for degradation products (Impurities A, C, and D) to ensure safety.
5. Why Use Ondansetron?
It blocks the nausea signal at the source.
Mechanism: It selectively blocks Serotonin (5-HT3) receptors. Chemotherapy/Surgery releases serotonin in the gut and brain, which triggers the “Vomiting Center.” Ondansetron blocks these receptors in both the gut (vagus nerve) and the brain (Chemoreceptor Trigger Zone), effectively stopping the urge to vomit.
Key Indications:
CINV: Chemotherapy-Induced Nausea & Vomiting.
RINV: Radiotherapy-Induced Nausea & Vomiting.
PONV: Post-Operative Nausea & Vomiting.
Pregnancy: (Off-label) Frequently used for Hyperemesis Gravidarum (severe morning sickness) when other drugs fail.
6. Export and Regulatory Support
We streamline the registration process for our B2B partners:
Dossier Support: We offer CTD and ACTD Dossiers for quick registration.
Certificates: Free Sale Certificate (FSC), COPP (WHO-GMP), and COA.
Logistics: Efficient shipping via Air or Sea (FOB Mumbai / CIF).
7. Frequently Asked Questions
Q: Who manufactures Ondansetron Tablets?
A: Healthy Life Pharma Pvt. Ltd. manufactures them in India.
Q: Does it make you sleepy?
A: Generally No. Unlike Promethazine or other older antiemetics, Ondansetron usually does not cause sedation, allowing patients to function normally.
Q: Is it safe for pregnancy?
A: It is widely used for severe morning sickness. While studies are generally positive, it should be used only if prescribed by a doctor (Risk vs. Benefit).
Q: Can I just swallow the MD tablet?
A: Yes. You can swallow it whole with water if you prefer. But it is designed to melt on the tongue for convenience.
CLINICAL PHARMACOLOGY & SAFETY INFORMATION
(For Registered Medical Practitioners & Patient Reference)
8. Dosage and Administration
Chemotherapy (Adults): 8mg taken 30 minutes before treatment, then 8mg every 8 hours.
Post-Operative: 16mg given 1 hour before anesthesia.
Administration:
FCT: Swallow whole with water.
MD: Peel back blister foil (do not push through). Place on tongue. Allow to dissolve. Swallow with saliva.
9. Side Effects and Precautions
Constipation: The most common side effect (due to slowed gut transit).
Headache: Common but manageable with analgesics.
QT Prolongation: Can affect heart rhythm. Use with caution in patients with electrolyte imbalances or existing heart conditions.
Serotonin Syndrome: Rare, but risk increases if combined with SSRIs/SNRIs.
10. Storage Instructions
Store below 30°C in a dry place.
Protect from light.