Description
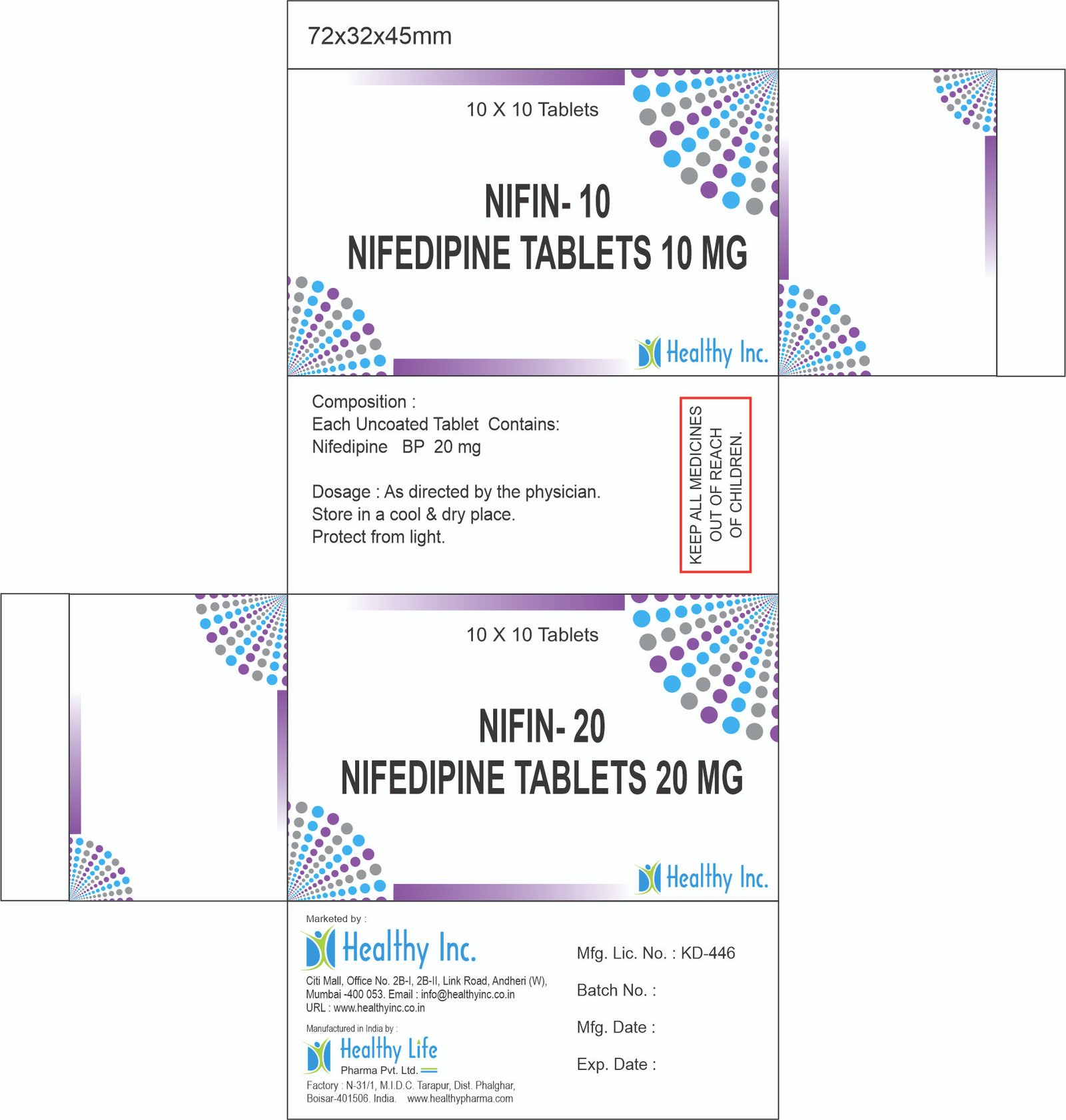
Nifedipine Tablets (Immediate Release) (5mg / 10mg)
Manufactured by: Healthy Life Pharma Pvt. Ltd. (WHO-GMP Certified)
Exported by: Healthy Inc (Star Export House)
1. Product Introduction
Healthy Life Pharma Pvt. Ltd. is a specialized Manufacturer of Nifedipine Immediate Release (IR) Tablets in India. Unlike the Sustained Release (SR) version used for long-term blood pressure control, these immediate-release tablets are designed for rapid absorption and are primarily indicated for the management of Chronic Stable Angina (chest pain) and vasospastic angina. They are also widely used off-label as a Tocolytic (to delay preterm labor) and for treating Raynaud’s Phenomenon.
We offer Contract Manufacturing (Third Party) services for this photosensitive drug. Nifedipine degrades rapidly upon exposure to light. Our WHO-GMP certified facility in Mumbai utilizes specialized light-shielded manufacturing suites (Sodium Vapor Lighting) and opaque coating technologies to ensure potency. Healthy Inc manages the export logistics, supplying hospitals and gynecology centers across Latin America, Southeast Asia, and Africa.
2. Product Specifications
| Parameter | Specification |
| Product Name | Nifedipine Tablets (Immediate Release) |
| Generic Name | Nifedipine Tablets USP / BP / IP |
| CAS Number | 21829-25-4 |
| Strength | 5mg / 10mg |
| Dosage Form | Film Coated or Sugar Coated Tablet (Yellow/Orange) |
| Standard | USP / BP / IP Compliant |
| Therapeutic Class | Calcium Channel Blocker / Anti-Anginal / Tocolytic |
| Shelf Life | 24 to 36 Months |
| Packaging | 10×10 Alu-Alu Blister / 100s Light-Resistant Bottle |
3. Manufacturing Technology
Precise light control is the manufacturing standard.
The Manufacturer: Healthy Life Pharma Pvt. Ltd.
Photosensitivity Protection: Nifedipine is extremely sensitive to UV light (daylight), converting into an inactive pyridine derivative within minutes. We manufacture the entire batch under Yellow Sodium-Vapor Lamps (no UV spectrum) to strictly prevent degradation.
Rapid Dissolution: For immediate release, we use super-disintegrants to ensure the tablet breaks down in the stomach within 15 minutes, allowing for quick onset of action (typically 20-30 minutes).
Coating: We use a robust Opaque Film Coating (Titanium Dioxide and Iron Oxide Yellow) to provide a permanent light barrier for the finished tablet.
The Exporter: Healthy Inc
High-Barrier Packaging: We exclusively recommend Alu-Alu Cold Form Blisters or Amber Blisters. Standard clear PVC blisters are not suitable for export as light exposure during retail display can ruin the product.
4. Quality Assurance
We adhere to strict Pharmacopoeial standards:
Dissolution: We test release profiles to ensure >80% of the drug releases within 20 minutes (Immediate Release standard).
Assay: We confirm the potency is strictly within 90-110% of the label claim.
Related Substances: We strictly monitor for the specific “Photolytic Impurity” (Nitrophenylpyridine analogue) to ensure the product has not been damaged by light.
5. Why Use Nifedipine IR?
It provides rapid vasodilation for specific conditions.
Mechanism: It blocks calcium channels in vascular smooth muscle, causing relaxation of coronary arteries (relieving angina) and peripheral arteries (improving circulation).
Key Indications:
Vasospastic Angina: (Prinzmetal’s Angina) Relieves spasms of the coronary arteries.
Raynaud’s Phenomenon: (Off-label) Relieves pain and blanching in fingers/toes by dilating capillaries.
Preterm Labor: (Off-label) Used to relax uterine muscles and delay birth for 48 hours to allow steroid administration for the baby’s lungs.
High Altitude Pulmonary Edema (HAPE): (Off-label) Used by climbers to lower lung pressure.
6. Export and Regulatory Support
We streamline the registration process for B2B partners:
Dossier Support: We offer CTD and ACTD Dossiers for quick registration.
Certificates: Free Sale Certificate (FSC), COPP (WHO-GMP), and COA.
Logistics: Efficient shipping via Air or Sea (FOB Mumbai / CIF).
7. Frequently Asked Questions
Q: Who manufactures Nifedipine IR Tablets?
A: Healthy Life Pharma Pvt. Ltd. manufactures them in India.
Q: Can I use this for high blood pressure?
A: CAUTION: Immediate-release Nifedipine is generally NOT recommended for long-term hypertension due to the risk of “Reflex Tachycardia” (racing heart) and erratic blood pressure swings. The Sustained Release (SR) version is preferred for BP control.
Q: Can I use it sublingually (under the tongue) for emergencies?
A: NO. This practice is now considered dangerous (risk of stroke/heart attack due to uncontrollable BP drop) and is strongly discouraged by FDA/WHO guidelines. Swallow whole.
Q: Is it safe for pregnancy?
A: It is widely used as a tocolytic (to stop labor) and for hypertension in pregnancy, but strictly under specialist medical supervision.
CLINICAL PHARMACOLOGY & SAFETY INFORMATION
(For Registered Medical Practitioners & Patient Reference)
8. Dosage and Administration
Angina: 10mg three times daily (TID).
Raynaud’s: 5mg to 20mg three times daily.
Administration: Swallow whole with water.
9. Side Effects and Precautions
Reflex Tachycardia: Rapid heartbeat as the body tries to compensate for the drop in blood pressure.
Flushing: Redness/warmth in the face.
Edema: Swelling of ankles/feet.
Hypotension: Dizziness or fainting.
Grapefruit Juice: Avoid. It increases the drug levels in the blood dangerously.
10. Storage Instructions
Store below 25°C in a dry place.
Protect from light. (Keep in the blister pack until the moment of use).