Description
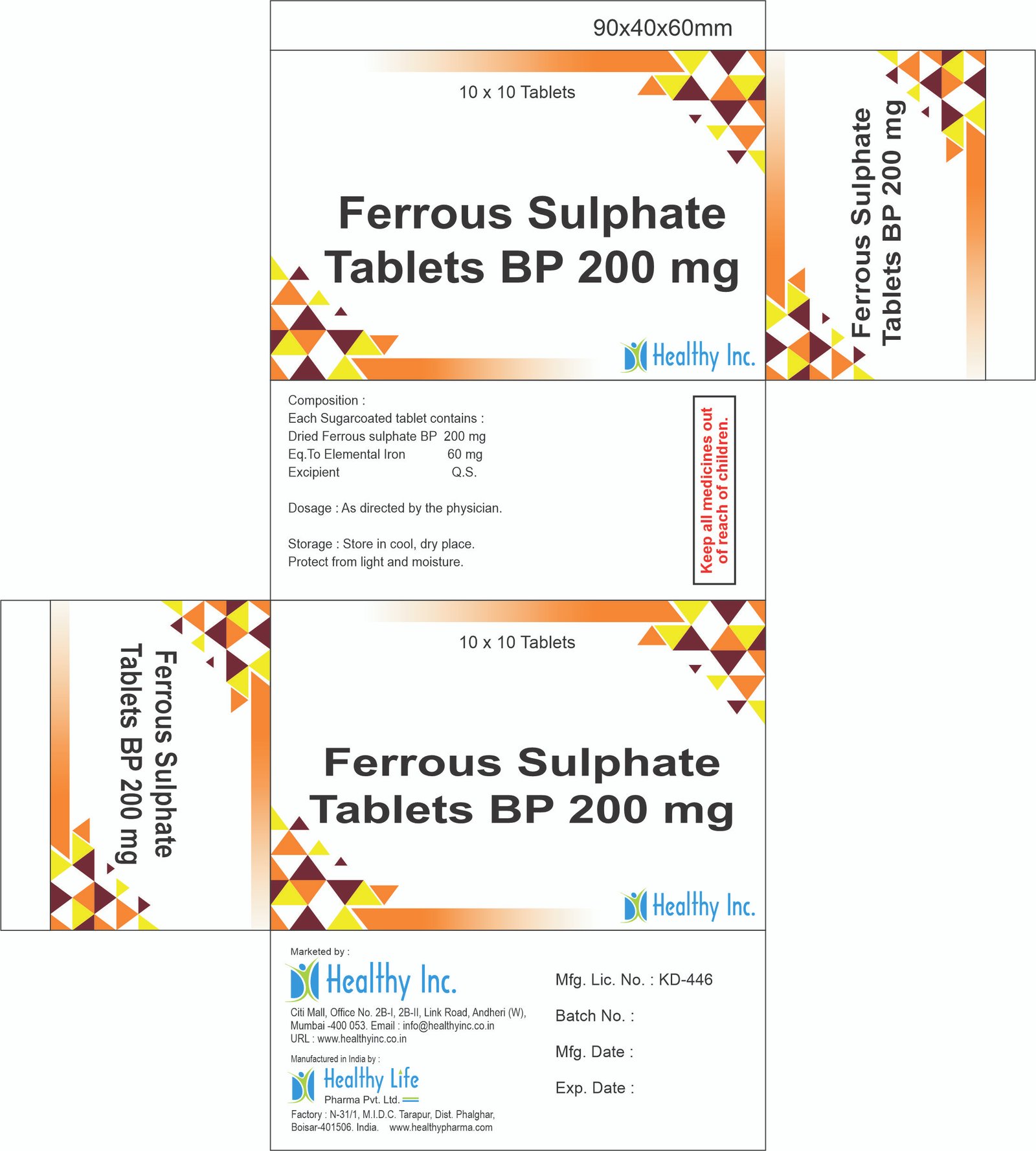
Ferrous Sulphate Tablets (200mg / 300mg)
Manufactured by: Healthy Life Pharma Pvt. Ltd. (WHO-GMP Certified)
Exported by: Healthy Inc (Star Export House)
1. Product Introduction
Healthy Life Pharma Pvt. Ltd. is a specialized Manufacturer of Ferrous Sulphate Tablets in India. Ferrous Sulphate is the “Gold Standard” for oral iron therapy. It is the most widely prescribed and cost-effective treatment for Iron Deficiency Anemia (IDA) globally. Despite the development of newer iron salts, Ferrous Sulphate remains the benchmark against which all other iron supplements are measured due to its high bioavailability.
We offer Contract Manufacturing (Third Party) services for this essential hematinic. Iron salts are highly susceptible to oxidation (turning from the active Ferrous form to the inactive Ferric form) and have a strong metallic taste. Our WHO-GMP certified facility in Mumbai utilizes traditional Sugar Coating (Red/Maroon) or advanced Enteric Coating to prevent gastric irritation, mask the taste, and ensure the iron remains stable in tropical climates. Healthy Inc manages the export logistics, supplying government health tenders, NGOs, and hospitals across Africa, Southeast Asia, and Latin America.
2. Product Specifications
| Parameter | Specification |
| Product Name | Ferrous Sulphate Tablets |
| Generic Name | Ferrous Sulphate Tablets USP / BP / IP |
| CAS Number | 7782-63-0 (Heptahydrate) / 7720-78-7 (Dried) |
| Strength | 200mg (Dried) / 300mg (Heptahydrate) |
| Elemental Iron | approx. 60mg to 65mg per tablet |
| Dosage Form | Sugar Coated or Enteric Coated Tablet (Red / Maroon) |
| Standard | USP / BP / IP Compliant |
| Therapeutic Class | Hematinic / Antianemic |
| Shelf Life | 24 to 36 Months |
| Packaging | 10×10 Blister / 1000s Bulk Jar |
3. Manufacturing Technology
Stability and compliance in every tablet.
The Manufacturer: Healthy Life Pharma Pvt. Ltd.
Oxidation Prevention: Ferrous iron ($Fe^{2+}$) is unstable and easily oxidizes to Ferric iron ($Fe^{3+}$) which is poorly absorbed. We use specific antioxidants and low-humidity processing zones to lock the iron in its active state.
Sugar Coating: To mask the unpleasant metallic taste and prevent nausea, we apply a multi-layered sugar coat. This makes the tablet smooth, sweet, and easy to swallow, significantly improving patient compliance.
Enteric Option: For patients with sensitive stomachs, we offer Enteric Coated tablets that bypass the stomach and release the iron in the small intestine, reducing gastric side effects like heartburn.
The Exporter: Healthy Inc
Tender Ready: We are specialists in fulfilling large-scale “Hospital Pack” orders (1000s HDPE Jars) required for national anemia control programs (e.g., WIFS) and maternal health initiatives.
4. Quality Assurance
We adhere to strict Pharmacopoeial standards:
Dissolution: We test release profiles to ensure the iron becomes available for absorption in the duodenum and upper jejunum.
Assay: We confirm the potency is strictly within 95-105% of the label claim.
Limit of Ferric Iron: We perform rigorous testing to ensure the level of oxidized (inactive) iron remains below pharmacopoeial limits (< 2%).
5. Why Use Ferrous Sulphate?
Restores the blood’s oxygen-carrying capacity.
Mechanism: Iron is an essential component of Hemoglobin, the protein in red blood cells that carries oxygen from the lungs to the tissues. Supplementation corrects the deficit in hemoglobin production, reversing symptoms of anemia like fatigue, breathlessness, and palpitations.
Key Indications:
Iron Deficiency Anemia: Treatment of established anemia due to blood loss, diet, or malabsorption.
Pregnancy: Prophylaxis to prevent anemia as blood volume expands.
Chronic Blood Loss: Menorrhagia (heavy periods) or GI bleeding.
6. Export and Regulatory Support
We streamline the registration process for our B2B partners:
Dossier Support: We offer CTD and ACTD Dossiers for quick registration.
Certificates: Free Sale Certificate (FSC), COPP (WHO-GMP), and COA.
Logistics: Efficient shipping via Air or Sea (FOB Mumbai / CIF).
7. Frequently Asked Questions
Q: Who manufactures Ferrous Sulphate Tablets?
A: Healthy Life Pharma Pvt. Ltd. manufactures them in India.
Q: Why is my stool black?
A: Do not panic. Unabsorbed iron turns stool black. This is a harmless and expected side effect of taking iron tablets.
Q: Can I take it with tea or coffee?
A: NO. Tannins in tea/coffee bind to iron and stop it from working. Wait at least 2 hours.
Q: What helps absorption?
A: Vitamin C. Taking the tablet with a glass of orange juice or lemon water significantly increases iron absorption.
CLINICAL PHARMACOLOGY & SAFETY INFORMATION
(For Registered Medical Practitioners & Patient Reference)
8. Dosage and Administration
Adults (Treatment): 200mg/300mg twice or three times daily.
Prophylaxis: 200mg/300mg once daily.
Administration: Best taken on an empty stomach (1 hour before meals). If gastric upset occurs, take with food (though this reduces absorption).
9. Side Effects and Precautions
Gastrointestinal: Nausea, abdominal pain, constipation, or diarrhea.
Teeth Staining: If the coating is broken in the mouth. Swallow whole.
Contraindications: Hemochromatosis (iron overload) and Hemolytic Anemia.
Interaction: Antacids, Calcium, Tetracyclines, and Levothyroxine reduce absorption. Separate doses by 2-4 hours.
10. Storage Instructions
Store below 25°C in a dry place.
Highly Hygroscopic. Keep container tightly closed to prevent the tablets from turning brown/sticky.