Description
Ondansetron Orally Disintegrating Tablets (4mg / 8mg)
Manufactured by: Healthy Life Pharma Pvt. Ltd. (WHO-GMP Certified)
Exported by: Healthy Inc (Star Export House)
1. Product Introduction
Healthy Life Pharma Pvt. Ltd. is a specialized Manufacturer of Ondansetron Orally Disintegrating Tablets (ODT) in India. This specialized dosage form is designed for immediate relief. Unlike standard tablets, these dissolve instantly on the tongue without the need for water, making them the ideal choice for patients suffering from severe nausea who cannot keep fluids down or have difficulty swallowing (dysphagia).
We offer Contract Manufacturing (Third Party) services for this high-tech formulation. Manufacturing ODTs requires precise humidity control and advanced taste-masking technology to ensure the bitter drug is palatable while maintaining a rapid disintegration time of less than 30 seconds. Healthy Inc manages the export logistics, supplying oncology clinics, pediatric centers, and hospitals across the USA, Europe, and emerging markets.
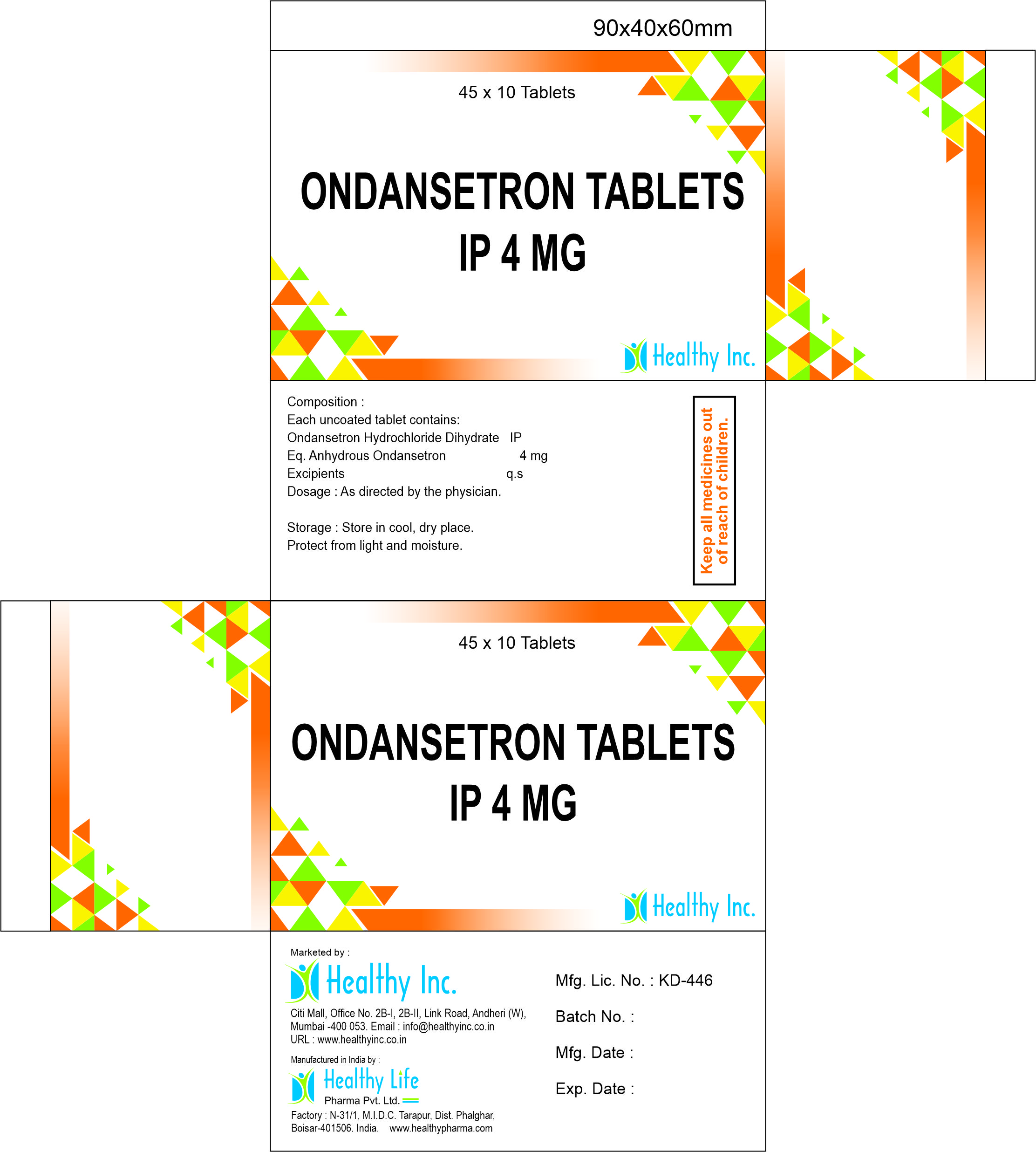
2. Product Specifications
| Parameter | Specification |
| Product Name | Ondansetron Orally Disintegrating Tablets |
| Generic Name | Ondansetron Orally Disintegrating Tablets USP / Ondansetron Mouth Dissolving Tablets |
| CAS Number | 99614-01-4 |
| Strength | 4mg / 8mg |
| Dosage Form | Orally Disintegrating Tablet (ODT) / Mouth Dissolving Tablet (MDT) |
| Standard | USP / BP / IP Compliant |
| Therapeutic Class | Antiemetic / 5-HT3 Antagonist |
| Shelf Life | 36 Months |
| Packaging | 10×10 Alu-Alu Blister (Peel-Push) |
3. Manufacturing Technology
Speed and taste are the defining features.
The Manufacturer: Healthy Life Pharma Pvt. Ltd.
Super-Disintegrant Matrix: We utilize a specialized blend of super-disintegrants (such as Crospovidone or Sodium Starch Glycolate) and highly soluble excipients (like Mannitol). This creates a porous matrix that wicks up saliva instantly, causing the tablet to fall apart in seconds.
Flavor & Taste Masking: Ondansetron is intensely bitter. We use Micro-Encapsulation or ion-exchange resin complexes to mask the bitterness without preventing the drug from working. We add premium flavors (Strawberry/Mint) to ensure patient acceptance, especially in children.
Humidity Control: ODTs are highly hygroscopic (they love moisture). We manufacture in Low-Humidity Suites (< 25% RH) to prevent the tablets from becoming soft or sticky during production.
The Exporter: Healthy Inc
Moisture Barrier Packaging: We strictly use Alu-Alu Cold Form Blisters. Standard PVC blisters allow too much moisture to pass through, which would ruin the rapid-dissolve feature of the tablet.
4. Quality Assurance
We adhere to strict Pharmacopoeial standards:
Disintegration Time: The critical test. Our benchmark is < 30 seconds in 0.1N HCl and Water.
Friability: ODTs are soft by design. We balance the hardness to ensure they don’t crumble in the pack (< 1% friability) but still melt in the mouth.
Assay: We confirm the potency is strictly within 90-110% of the label claim.
5. Why Use Ondansetron ODT?
It offers “Compliance” when patients are vomiting.
Convenience: No water needed. This is vital for patients who vomit immediately after drinking fluids.
Pediatrics & Geriatrics: Perfect for children or elderly patients who cannot or will not swallow whole tablets.
Rapid Onset: Because it disperses immediately, absorption can begin slightly faster, providing quicker relief.
6. Export and Regulatory Support
We streamline the registration process for our B2B partners:
Dossier Support: We offer CTD and ACTD Dossiers (with Stability Data) for quick registration.
Certificates: Free Sale Certificate (FSC), COPP (WHO-GMP), and COA.
Logistics: Efficient shipping via Air or Sea (FOB Mumbai / CIF).
7. Frequently Asked Questions
Q: Who manufactures Ondansetron ODT?
A: Healthy Life Pharma Pvt. Ltd. manufactures them in India using specialized ODT technology.
Q: Do I chew it?
A: No. Just place it on the tip of your tongue. It will dissolve in seconds. Then swallow the saliva.
Q: Does it taste bad?
A: No. We use advanced taste-masking and flavoring (usually Strawberry or Mint) to make it pleasant.
Q: Why is the packaging so thick?
A: It is Alu-Alu foil. It protects the tablet from moisture in the air, which would otherwise make it dissolve inside the packet.
CLINICAL PHARMACOLOGY & SAFETY INFORMATION
(For Registered Medical Practitioners & Patient Reference)
8. Dosage and Administration
Adults (Chemotherapy): 8mg ODT taken 30 minutes before treatment.
Children (4-11 years): 4mg ODT taken 30 minutes before treatment.
Post-Operative: 16mg ODT given 1 hour before anesthesia.
Administration: Do not push the tablet through the foil. Peel back the foil lid, gently remove the tablet with dry hands, and place immediately on the tongue.
9. Side Effects and Precautions
Phenylketonurics: Warning: Some ODT formulations contain Aspartame (a source of Phenylalanine). Check the label if you have PKU.
Constipation: Common side effect.
Headache: Common side effect.
QT Prolongation: Use with caution in patients with heart rhythm disorders.
10. Storage Instructions
Store below 30°C in a dry place.
Keep in original blister until administration. Exposure to air will degrade the tablet rapidly.