Description
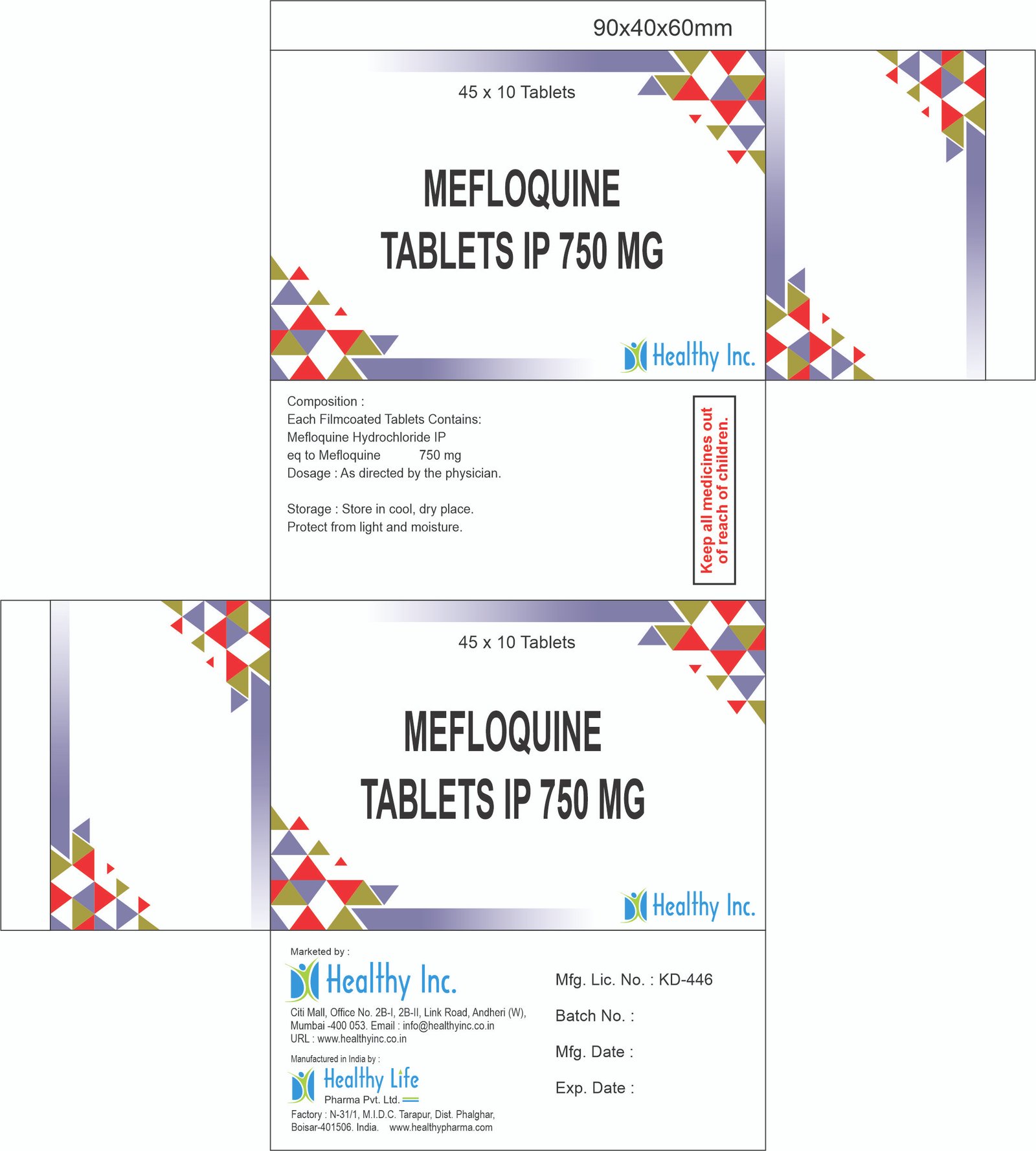
Mefloquine Tablets (250mg)
Manufactured by: Healthy Life Pharma Pvt. Ltd. (WHO-GMP Certified)
Exported by: Healthy Inc (Star Export House)
1. Product Introduction
Healthy Life Pharma Pvt. Ltd. is a specialized Manufacturer of Mefloquine Hydrochloride Tablets in India. Mefloquine is a potent Antimalarial Drug (methanolquinoline derivative) used for both the Treatment and Prophylaxis (Prevention) of malaria. It is particularly valuable for treating Plasmodium falciparum malaria in regions where the parasite has developed resistance to older drugs like Chloroquine.
We offer Contract Manufacturing (Third Party) services for this critical tropical medicine. Mefloquine is intensely bitter and has poor water solubility. Our WHO-GMP certified facility in Mumbai utilizes advanced Micronization and Taste-Masking technologies to ensure the tablet dissolves effectively in the gut while minimizing the unpleasant taste during swallowing. Healthy Inc manages the export logistics, supplying peacekeepers, travelers, and government health programs across Malaria-endemic zones in Africa and Southeast Asia.
2. Product Specifications
| Parameter | Specification |
| Product Name | Mefloquine Tablets |
| Generic Name | Mefloquine Hydrochloride Tablets USP / BP / IP |
| CAS Number | 53230-10-7 (Hydrochloride) |
| Strength | 250mg |
| Dosage Form | Uncoated Tablet (White / Round / Cross-Scored) |
| Standard | USP / BP / IP Compliant |
| Therapeutic Class | Antimalarial |
| Shelf Life | 24 to 36 Months |
| Packaging | 10×10 Blister / 6s, 4s Consumer Pack (Travel Pack) |
3. Manufacturing Technology
Bioavailability and compliance are the challenges.
The Manufacturer: Healthy Life Pharma Pvt. Ltd.
Micronization: Mefloquine is a hydrophobic drug. We use Micronized API to increase the specific surface area, ensuring the drug dissolves consistently in the stomach fluids to achieve therapeutic blood levels.
Taste Masking: The drug is chemically related to quinine and is extremely bitter. We use specialized film-coating polymers or sweeteners in the granulation stage to mask the bitterness, which is crucial for preventing vomiting (which would lead to dose failure).
Dissolution: We optimize the disintegration time to ensure rapid release, which is vital for acute treatment.
The Exporter: Healthy Inc
Compliance Packaging: For travelers, we offer Wallet Packs (4 or 6 tablets) with printed dosage schedules (e.g., “Week 1,” “Week 2”) to ensure they stick to the weekly prophylaxis regimen.
4. Quality Assurance
We adhere to strict Pharmacopoeial standards:
Dissolution: We test release profiles to ensure >75% of the drug releases within 30 minutes.
Assay: We confirm the potency is strictly within 90-110% of the label claim.
Impurity Profiling: We strictly monitor for degradation products to ensure safety over the shelf life.
5. Why Use Mefloquine?
It is the long-acting shield against resistant malaria.
Mechanism: It acts by interfering with the parasite’s ability to metabolize hemoglobin. It causes the accumulation of toxic heme within the parasite, eventually killing it.
Key Indications:
Prophylaxis: Prevention of malaria for travelers visiting endemic areas (taken once weekly).
Treatment: Cure of mild-to-moderate acute malaria caused by P. falciparum or P. vivax.
Advantage: Its long half-life (2-3 weeks) allows for convenient Once-Weekly Dosing for prevention, unlike Doxycycline (daily) or Atovaquone (daily).
6. Export and Regulatory Support
We streamline the registration process for our B2B partners:
Dossier Support: We offer CTD and ACTD Dossiers for quick registration.
Certificates: Free Sale Certificate (FSC), COPP (WHO-GMP), and COA.
Logistics: Efficient shipping via Air or Sea (FOB Mumbai / CIF).
7. Frequently Asked Questions
Q: Who manufactures Mefloquine Tablets?
A: Healthy Life Pharma Pvt. Ltd. manufactures them in India.
Q: Is it Lariam?
A: Lariam is the brand name (Roche). We manufacture the Generic Equivalent (Mefloquine HCl), which is bioequivalent and cost-effective.
Q: When do I start taking it for travel?
A: You must start taking it 1 to 2 weeks before arrival in the malaria zone, continue during the stay, and for 4 weeks after leaving.
Q: Does it cause bad dreams?
A: Yes. Vivid dreams, anxiety, and insomnia are known side effects. It affects the central nervous system.
CLINICAL PHARMACOLOGY & SAFETY INFORMATION
(For Registered Medical Practitioners & Patient Reference)
8. Dosage and Administration
Prophylaxis (Prevention): 250mg once weekly. Take on the same day each week.
Treatment (Acute Malaria): Total dose of 1250mg (5 tablets) usually split as:
Initial: 750mg (3 tabs).
6-8 hours later: 500mg (2 tabs).
Administration: Take with food and a full glass of water to reduce stomach upset.
9. Side Effects and Precautions
Neuropsychiatric Effects: Boxed Warning: Can cause dizziness, anxiety, paranoia, hallucinations, and depression. Contraindicated in patients with a history of depression, anxiety disorders, or psychosis.
Seizures: Contraindicated in patients with a history of epilepsy/seizures.
Cardiac: Use with caution in patients with cardiac conduction disorders.
Vestibular: Dizziness and loss of balance (vertigo). Do not drive if affected.
10. Storage Instructions
Store below 25°C in a dry place.
Protect from light and moisture.