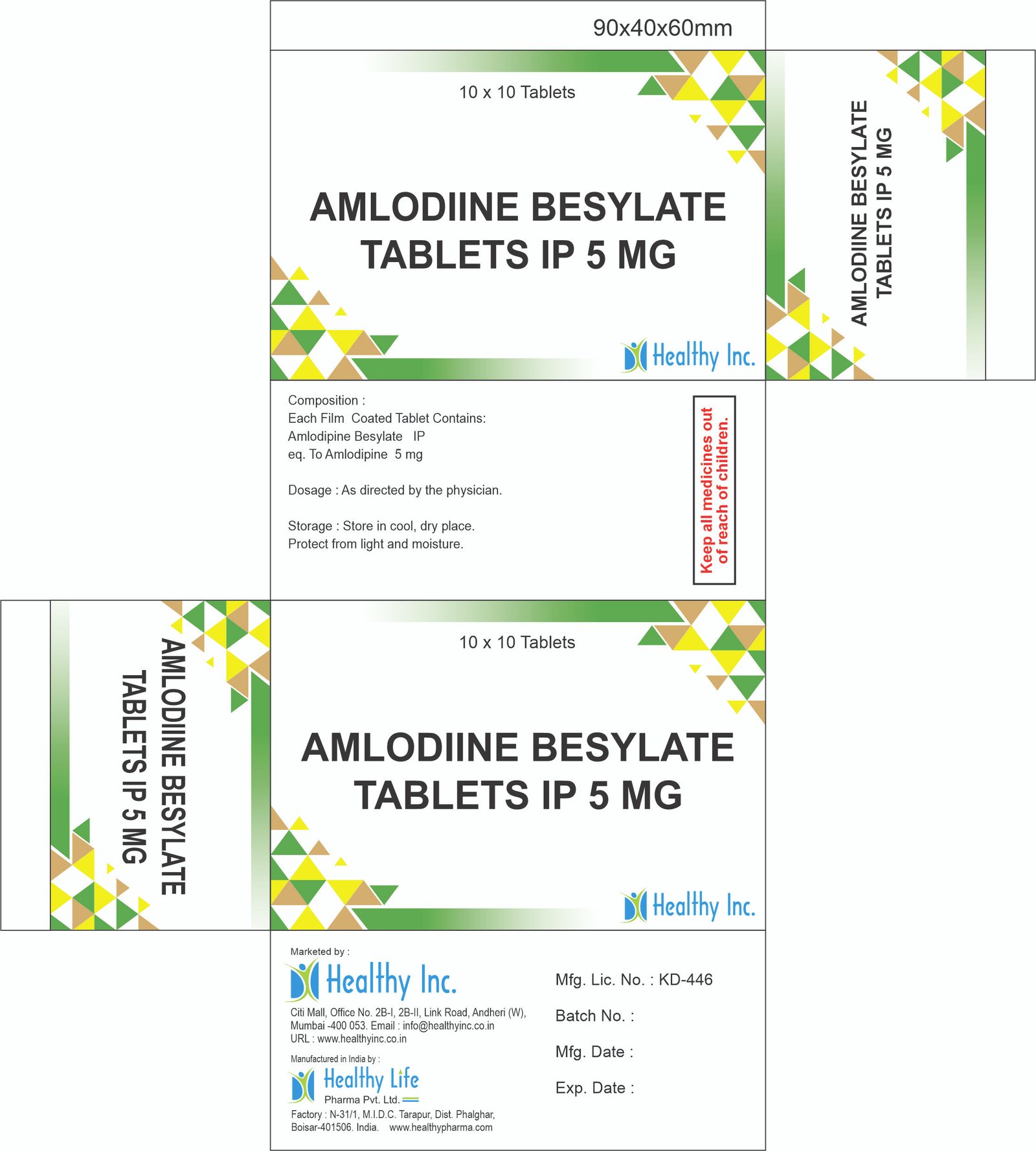
Description
Losartan Potassium & Amlodipine Besylate Tablets
(50mg/5mg | 100mg/5mg | 50mg/10mg)
Manufactured by: Healthy Life Pharma Pvt. Ltd. (WHO-GMP Certified)
Exported by: Healthy Inc (Star Export House)
1. Product Introduction
Healthy Life Pharma Pvt. Ltd. is a specialized Manufacturer of Losartan Potassium and Amlodipine Besylate Tablets in India. This powerful Fixed-Dose Combination (FDC) brings together two of the world’s most trusted antihypertensives: Losartan (an Angiotensin Receptor Blocker – ARB) and Amlodipine (a Calcium Channel Blocker – CCB).
By attacking high blood pressure through two distinct mechanisms simultaneously, this combination offers superior efficacy for patients whose blood pressure is uncontrolled on monotherapy. It also helps improve patient compliance by reducing the “pill burden”—combining two daily pills into one. We offer Contract Manufacturing (Third Party) services for this product, utilizing advanced bilayer or homogenous compression technology at our WHO-GMP certified facility in Mumbai. Healthy Inc manages the export logistics, supplying cardiology distributors and hospital tenders across the CIS region, Southeast Asia, and Latin America.
2. Product Specifications
| Parameter | Specification |
| Product Name | Losartan Potassium & Amlodipine Besylate Tablets |
| Generic Name | Losartan Potassium and Amlodipine Tablets USP / BP / IP |
| Composition | Losartan 50mg + Amlodipine 5mg (Standard)
Losartan 100mg + Amlodipine 5mg
Losartan 50mg + Amlodipine 10mg |
| Active Salts | Losartan Potassium + Amlodipine Besylate |
| Dosage Form | Film Coated Tablet (White / Yellow / Round / Oval) |
| Standard | USP / BP / IP Compliant |
| Therapeutic Class | Antihypertensive (ARB + CCB Combination) |
| Shelf Life | 24 to 36 Months |
| Packaging | 10×10 Alu-Alu Blister / 30s, 100s Bottle |
3. Manufacturing Technology
Two pathways, one pill.
The Manufacturer: Healthy Life Pharma Pvt. Ltd.
Stabilization: Losartan is hygroscopic (absorbs moisture), while Amlodipine is photosensitive (degrades in light). We use a specialized formulation process that stabilizes both molecules, often employing Alu-Alu Cold Form Blistering to provide a complete barrier against both humidity and UV light.
Nitrosamine-Free: We strictly source Losartan API that is certified free from “Sartan” impurities (Nitrosamines), ensuring the product meets the highest global safety standards (USFDA/EMA guidelines).
Dissolution Synergy: Our formulation is engineered to release both drugs at a synchronized rate, ensuring that the peak effect of vasodilation occurs smoothly, preventing sudden drops in blood pressure (hypotension).
The Exporter: Healthy Inc
Market Adaptation: We can customize the packaging language and trade dress to match the regulatory requirements of specific territories (e.g., Spanish for LATAM, Russian for CIS).
4. Quality Assurance
We adhere to strict Pharmacopoeial standards:
Assay: We confirm the potency of both actives is strictly within 90-110% of the label claim.
Related Substances: Rigorous HPLC testing is conducted to monitor for Amlodipine oxidative impurities and Losartan degradation products.
Uniformity: Automated weight variation controls ensure every tablet contains the precise ratio of 50mg/5mg.
5. Why Use Losartan + Amlodipine?
Double the vasodilation, fewer side effects.
Mechanism:
Losartan (The ARB): Blocks the action of Angiotensin II, a chemical that tightens blood vessels. This relaxes the vessels.
Amlodipine (The CCB): Blocks calcium from entering the muscle cells of the heart and arteries. This prevents the arteries from constricting.
Synergy: By working on two different pathways, they lower blood pressure more effectively than either drug alone. Additionally, Losartan can help reduce the leg swelling (edema) that is a common side effect of Amlodipine.
Key Indications:
Essential Hypertension: For patients not adequately controlled on Losartan or Amlodipine alone.
Severe Hypertension: Initial therapy for patients likely to need multiple drugs to reach their BP goal.
6. Export and Regulatory Support
We streamline the registration process for our B2B partners:
Dossier Support: We offer CTD and ACTD Dossiers for quick registration.
Certificates: Free Sale Certificate (FSC), COPP (WHO-GMP), and COA.
Logistics: Efficient shipping via Air or Sea (FOB Mumbai / CIF).
7. Frequently Asked Questions
Q: Who manufactures Losartan Amlodipine Tablets?
A: Healthy Life Pharma Pvt. Ltd. manufactures them in India.
Q: Is it safe to take two drugs in one?
A: Yes. Fixed-Dose Combinations (FDCs) are standard practice in cardiology. They improve compliance because you only have to remember one pill instead of two.
Q: Will my feet swell?
A: Less likely. Amlodipine alone often causes ankle swelling (edema). Adding Losartan helps dilate veins as well, often reducing this swelling compared to taking Amlodipine alone.
Q: When should I take it?
A: In the morning. It provides 24-hour control. Taking it at the same time every day helps you remember.
CLINICAL PHARMACOLOGY & SAFETY INFORMATION
(For Registered Medical Practitioners & Patient Reference)
8. Dosage and Administration
Dose: One tablet daily.
Titration: Treatment is usually started with the 50mg/5mg strength. If BP remains uncontrolled after 2-4 weeks, the dose may be titrated up to 50mg/10mg or 100mg/5mg.
Administration: Swallow whole with water. Can be taken with or without food.
9. Side Effects and Precautions
Common: Dizziness, headache, peripheral edema (swelling of ankles), palpitations.
Hypotension: Watch for lightheadedness when standing up (orthostatic hypotension), especially in elderly patients.
Pregnancy: CONTRAINDICATED. Losartan can cause severe injury or death to the developing fetus. Discontinue immediately if pregnancy is detected.
Liver/Kidney: Use with caution in patients with hepatic or renal impairment.
10. Storage Instructions
Store below 25°C in a dry place.
Protect from light and moisture. (Keep in the original blister).